Abstract
Background: Nucleophosmin (NPM1) gene mutations are rarely detected in patients with non-acute myeloid neoplasms (MN), such as myelodysplastic syndromes (MDS) and chronic myelomonocytic leukemia (CMML). Retrospective case series suggested that they are typically associated with an aggressive disease course and progress into acute myeloid leukemia (AML) in a short period of time, representing a distinct clinicopathological entity. However, the long-term outcomes and prognostic factors of these MNs in the era of modern therapies remain not well defined given their rarity.
Methods: In the current study, we retrospectively reviewed clinical features, treatments, and outcomes of patients with NPM1-mutated MDS or CMML seen at Moffitt Cancer Center between 2009 and 2022. In comparison, AML patients with confirmed prior histories of MDS or CMML (sAML) that had NPM1 mutations detected at their AML diagnoses were used as the control group.
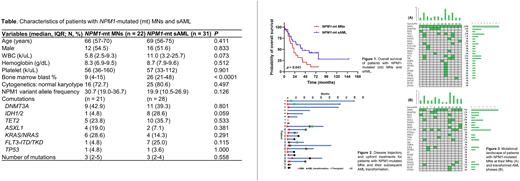
Results: We identified 22 patients with NPM1-mutated MNs (median age 66 years at diagnosis and 54.5% male), including 16 cases of MDS (68.8% excess blasts) and 6 cases of CMML (50% CMML-2). Most patients had a normal karyotype (72.7%), and the median variant allele frequency of NPM1 mutation was 30.7%. Compared to NPM1-mutated sAML (n = 31), patients with NPM1-mutated MNs had lower bone marrow blast counts as expected and a trend for less IDH1/2 and FLT3-ITD/TKD mutations (Table). The majority of NPM1-mutated MNs (77.3%) were treated with upfront hypomethylating agents, and treatment response was unsatisfactory, with only one complete response. Four patients ultimately underwent allogeneic stem cell transplant. In contrast, most NPM1-mutated sAML (71.0%) received intensive chemotherapy as the frontline therapy, which resulted in a higher rate of complete response (81.8%), and 12 patients (38.7%) subsequently underwent allogeneic stem cell transplant. The overall survival was significantly inferior in NPM1-mutated MNs compared to sAML (median 22.1 vs. 41 months, p = 0.041; p = 0.023 if data censored at the time of transplant), which remained significant after adjusted by age (hazard ratio 2.15, 95% confidence interval 1.07-4.34, p = 0.032). During follow-up, 14 patients (63.6%) with NPM1-mutated MNs transformed into AML with a median time to transformation of 21.1 months. Among them, nine patients were able to receive intensive chemotherapy as induction for AML, and four achieved complete responses. In patients with mutational data available at both MN and subsequent AML phases, NPM1 mutations remained detectable in 75% of cases, and gain of FLT3-ITD was commonly seen (41.7%) at the AML phase. In terms of prognostic factors, concurrent baseline DNMT3A mutations conferred a trend of inferior outcomes (median 16.0 vs. 30.7 months; p = 0.116), and patients who received transplant showed improved outcomes (median 59.7 vs. 18.1 months; p = 0.029).
Conclusion: Our findings suggest that NPM1-mutated MNs are an aggressive clinicopathological entity and support the recent WHO proposal to reclassify these MNs into AML irrespective of the blast counts, as they may benefit from more intensive treatment options.
Disclosures
Kuykendall:Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Protagonist: Other: Research Support; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. Padron:Blueprint: Honoraria; Incyte: Research Funding; Kura: Research Funding; Stemline: Honoraria; Taiho: Honoraria; BMS: Research Funding; Syntrix Pharmaceuticals: Research Funding. Sweet:berGenBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Syntrix Pharmaceuticals: Research Funding; Incyte: Research Funding; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Mablytics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AROG: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Lancet:Syntrix Pharmaceuticals: Research Funding; Novartis: Consultancy; Dava Oncology: Consultancy; Jasper Therapeutics: Consultancy; Dedham Group: Consultancy; Servier: Consultancy; Agios/Servio: Consultancy; Boxer Capital: Consultancy; Astellas: Consultancy; Jazz: Consultancy; BerGenBio: Consultancy; Millenium Pharma/Takeda: Consultancy; ElevateBio Management: Consultancy; Daiichi Sankyo: Consultancy; Celgene/BMS: Research Funding; AbbVie: Consultancy. Komrokji:Geron: Consultancy; PharmaEssentia, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Taiho Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acceleron Pharma: Consultancy; Servier: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Speakers Bureau; CTI BioPharma, Innovent: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Sallman:Nemucore: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Intellia: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Kite: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Lixte: Patents & Royalties: LB-100; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Syntrix Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal